The Central Sterilizing Service Department
The Sterile Processing Department (SPD), also known as the Central Sterile Services Department (CSSD) is an integrated activity of the utmost importance in healthcare facilities. The purpose of the CSSD is infection control and safeguarding the health of patients undergoing diagnostic investigations or surgery and therapy.
The Central Service, plays a vital role in all procedures and processes related to the activity of the operating theatres and wards of any hospital.
The CSSD is essential in:
the supply of sterile products, consisting of RMDs (reusable medical devices) needed in all healthcare activities.

the application of all possible strategies for increasing patient safety and ensuring a better prevention against healthcare-associated infections (HAIs).

the comprehensive tracking of the sterile process, so that it takes place in the correct sequence of implementation and that the history of each medical device can be traced at any time
The reprocessing of surgical instruments is the end result of a process that, through standardised, repeatable, documentable and traceable methods, allows to eliminate every microorganism from surgical tools and medical equipment after their use.
Manual
pre-treatment

Washing and thermal disinfection

Packaging and sterilization

Storage
and reuse

In addition to the manual equipment present for pre-treatment and packaging of surgical instruments, a CSSD uses a wide range of washing and disinfecting equipment for reusable medical devices [link alla pagina lavaggio] as well as different types of machines for high temperature and low temperature sterilization link alla pagina sterilizzazione], the latter being reserved for all types of medical devices that cannot be sterilized with steam.

RED ZONE DIRTY AREA |
A wide variety of surgical instruments and other dirty and used medical devices coming from the operating theatres (OT) of all hospital wards are transported inside containers and baskets to the CSSD’s Septic Area. It is essential to implement a traceability software that tracks the journey of the surgical kits. The initial reprocessing stages of reusable medical devices may include manual pre-washing and other treatments prior to the washing and sterilization stages. |

BLUE ZONE CLEAN AREA |
Automated cleaning and disinfection make the surgical instruments in the containers and baskets safe and easy to handle. In the Aseptic Area of the CSSD, reusable medical devices are sorted, inspected and packaged before undergoing the most appropriate sterilization cycle. |

GREEN ZONE STERILE AREA |
At the end of the sterilization treatment, the instruments arrive in the sterile area of the CSSD. Here the RMDs are stored in warehouses or transported back to the wards and operating theatres for reuse. |

The CSSD is where disinfection machines and sterilization autoclaves reprocess all surgical instruments in compliance with standards, technical reports, guidelines and procedures aimed at ensuring the safety of operators and patients.
A large number of reusable medical devices and surgical sets from operating theatres and surgeries are processed daily in a CSSD. Each reusable medical device is washed, packaged and sterilized through procedures aimed at making it sterile and keeping it that way until it is used again.
Inside the Sterile Processing Department (SPD), the procedure is carried out by a number of professionals, with appropriate equipment and technology protecting them and the facility from any risk, safeguarding the health of patients from the risk of healthcare-associated infections (HAI).
Cisa Group, a leader in the field of infection control and sterilization machines for hospitals, develops technologies to fight healthcare-associated infections (HAI) and offers complete turnkey solutions for the installation of hospital Sterile Processing Departments (SPDs).
The standard offer for CSSDs is available in three different packages:

5 Operating Theatres 15.000 - 21.000 U.S./year 6.000 - 8.500 operations/year 260 days/year 6 - 8 cycles/day Show more |

10 Operating Theatres 30.000 - 42.000 U.S./year 12.000 - 17.000 operations/year 260 days/year 6 - 8 cycles/day Show more |

20 Operating Theatres 64.000 - 84.000 U.S./year 24.000 - 34.000 operations/year 260 days/year 6 - 8 cycles/day Show more |
The size of sterilization and washing disinfection equipment is one thing, but the space required for their installation or maintenance in a hospital CSSD is quite another.
Analyzing the layout and overall dimensions of CSSD machinery is crucial to achieving an efficient CSSD design.

Every Cisa Group CSSD package guarantees a total amount of saved water compared to the average market value of consumption.
The total value is referred to machines working 260 days/year, 8 cycles per day.
The total water savings obtained thanks to the use of the Aquazero® vacuum pump [photo] instead of the standard liquid ring vacuum pump and the use of the new generation of Cisa Group washer-disinfectors is significant.

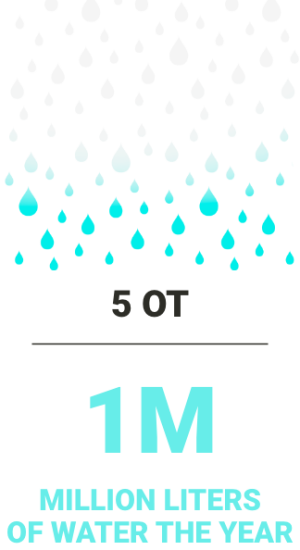


Water is considered essential in a hospital CSSD.
Water is the indispensable fuel for the whole reprocessing process of reusable medical devices (RMDs) in every hospital CSSD. The water used in a CSSD is said to have three costs. What does this mean?


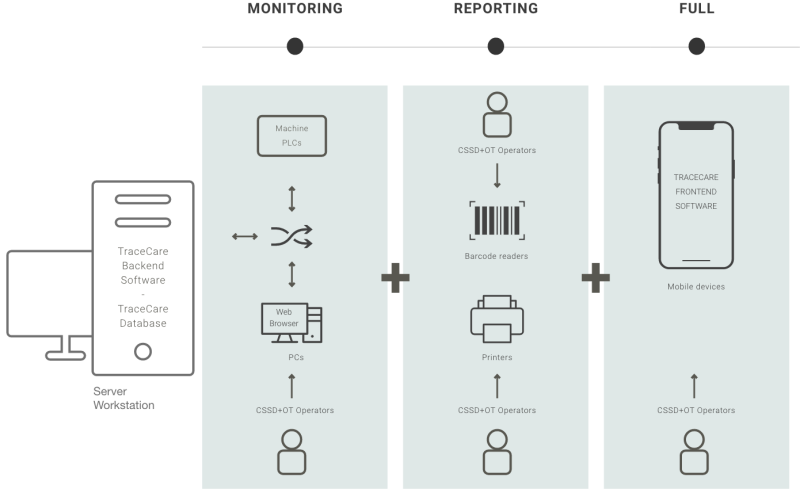
Tracecare® is the traceability system developed by Cisa Group, which in its complete version allows to manage and monitor the kit’s process through the areas (dirty area, clean area, sterile area) that make up the CSSD, facilitating the reduction of clinical risk.
The CSSD reprocesses a large number of RMDs, surgical instruments and surgical sets every day and it cannot afford inefficiencies and production stops.
The task of the CSSD is of the utmost importance for the control of clinical risk, the fight against HAIs and for the maximum safety of patients and healthcare staff.