

Cisa Group’s Service is a form of assistance that goes beyond the typical after-sales services: not only maintenance and spare parts, therefore, but a full and complete support able to guide the Clinical Engineering Services from the early stages of a sterilization project, during the installation of the machinery in the CSSD and over the years.
The professionalism and technological and process skills that are made available to customers during each project are the real added value.
Customer care starts in the design stage: each request is processed by a diversified team working in synergy to find suitable solutions for each request.


Assistance, tests and checks, support to clinical engineering and operational technicians, organising and programming training for operational staff.

Maintenance, assistance, original spare parts. Updates of IT tools and planning of specific training activities, with customized options.
SPECIALISED FIELD TECHNICIANS

HELP DESK SPECIALISTS

PIECES OF EQUIPMENT MAINTAINED
INTERVENTIONS PER YEAR

Assistance in Italy: assistenza@cisagroup.it
Assistance Abroad: service@cisagroup.it
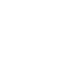
The Remote Maintenance System (RMS) is the function that allows to perform remote maintenance on steam autoclaves and washer-disinfectors.
Using an Ethernet network with Internet access, the RMS system allows the technical service to connect to the sterilization equipment and to washer-disinfectors and perform any necessary service.
Through remote display of the equipment’s touchscreen, the technician can access:

all its functions

cycle history

alarms history

the current cycle

update the touchscreen interface

update the PLC software
The RMS provides real-time problem diagnosis, thus allowing for a timely resolution, reducing the downtime of the sterilizer or washer-disinfector and avoiding any delays in the operation of the hospital’s CSSD.
The Service also takes care of supplying original spare parts for Cisa Group machines, maintenance kits and consumables, in order to guarantee their correct functioning and the best performance over time, thus ensuring the proper operation of the entire sterilization process.
Assistance for spare parts: sparesparts@cisagroup.it

Support for commissioning Validations and qualifications Electrical safety verification Thermometric testing Residual moisture testing Technical consultancy Support for notified body audits Feeler gauge testing Support for environmental testing and analysis |
Predictive maintenance Preventive maintenance Corrective maintenance Field service Remote assistance Business Intelligence Original spare parts |
The Clinical Engineering Service CES (or biomedical technology management service) encompasses many activities and responsibilities within a local healthcare service and takes on specific characteristics according to the type of organisation and processes in which it is embedded.
The requirements of modern healthcare management have deeply changed. Clinical Engineering deals with identifying needs and defining the programmes and directions of the local healthcare service: it applies engineering concepts and technologies to the healthcare service and the technologies in use, including IT services. Clinical Engineering was set up to guarantee a high degree of safety and compliance of electromedical equipment, manage its maintenance and support the healthcare service in planning the purchase of new equipment.
 |
1. Clinical Engineers (engineering staff) |
 |
2. Biomedical and general electromedical equipment technicians (technical staff) |
 |
3. Secretarial and accounting services (administrative staff) |
A Clinical Engineering Service is organized and sized in order to properly carry out all the activities assigned to it within the healthcare facility. Put simply, the main tasks of the CES can be split into two macro-areas:

organization, coordination, programming,

definition and expenditure of the healthcare facility’s budget

the maintenance and testing
of equipment
One of the CES‘s main tasks is undoubtedly to support the programming and planning of investments and the definition of a procurement plan for technological upgrade. Its activities are always in line with the economic constraints related to the budget and to relevant regulations. The application of “Health Technology Assessment” (HTA) methodologies allows it to consider and integrate multiple technical and organisational aspects.
When a healthcare facility is considering the purchase of new biomedical technologies, the Clinical Engineering Service, given its specific expertise, is responsible for preparing all the technical and regulatory acts relating to the investment. The Clinical Engineering Service works on drawing up technical specifications, conducts continuous market surveys, drafts specifications and evaluates bids.
The Clinical Engineering Service is the entity with the greatest knowledge of biomedical technologies available on the market, including technologies relating to the sterilization process; it also has a clear picture of the current situation in its own facility. It manages the database of technologies in use at the company and knows their actual state of use. Thanks to the unequivocal identification of each device in use, it has data on the personal, technical and performance information of all electromedical equipment and this allows it to always have under control the current status and keep track of any changes or what needs to be renewed.
In practice, by managing the whole technology assets, the CES must ensure the efficiency, effectiveness and safety status of the biomedical equipment over time, in accordance with the provisions of the specific technical regulations. It is therefore responsible for acceptance testing, test trials and safety checks, and for the formalization of all internal procedures concerning order processing, taking charge of the equipment and encoding its data in the company systems.
The Clinical Engineering Service will follow the technology over time, monitoring its activity by scheduling corrective and extraordinary maintenance, preventive maintenance, and calibrations and adjustments in accordance with the user manual or with specific regulations, as well as electrical safety checks, functional checks and any retrofitting for compliance.
The CES is in fact responsible for managing use-related risk, and regulatory compliance of technologies used is a key aspect. Its detailed knowledge of the intrinsic hazards of the different types of equipment and information on their state of use, maintenance and obsolescence allow it to ensure the functionality and quality of the equipment and to provide the necessary information for risk assessment, in support of the Prevention and Protection Service.
In conclusion, a trusted partner such as a market-leading manufacturer with extensive experience in the development and maintenance of healthcare sterilization machinery can always be of use to a Clinical Engineering Service that, in summary, takes care of:
 |
risk assessment for healthcare workers and patients |
 |
calculating the technological obsolescence of existing machines |
 |
calculating the workload of existing technologies |
 |
knowing the care network and the diagnostic processes and pathways in which new technologies are to included |
 |
decision making, with full knowledge of the regulatory framework. |
phone +39 0583 15381 EMEA MARKET
phone +55 47380 19090 LATAM MARKET
